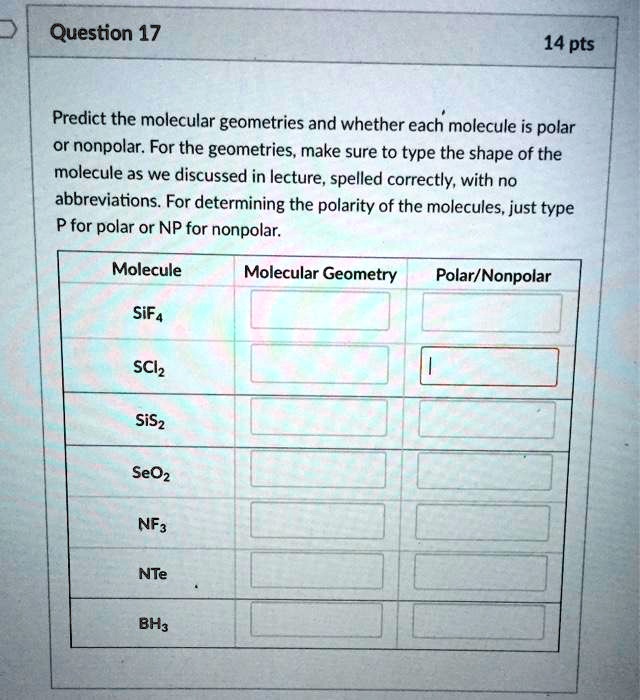
POLARITY. Polar Molecules l A polar molecule is a molecule with one end having positive electrical charge and the other end having negative electrical. - ppt download
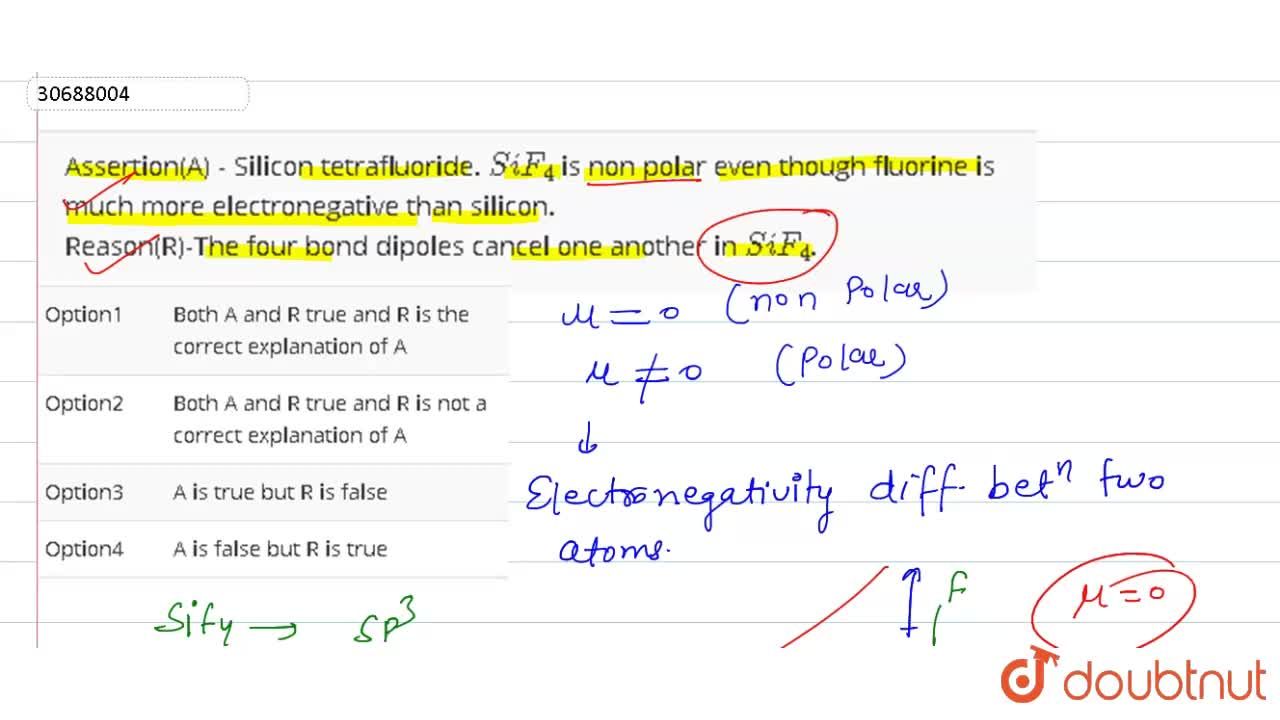
is non polar even though fluorine is much more electronegative than silicon. Reason(R)-The four bond dipoles cancel one another in
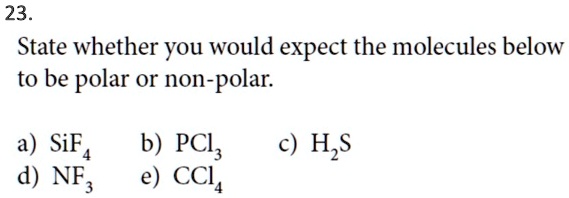
SOLVED: 23 State whether you would expect the molecules below to be polar or non-polar: a) SiF4 d) NF, b) PCl; e) CCL H,S

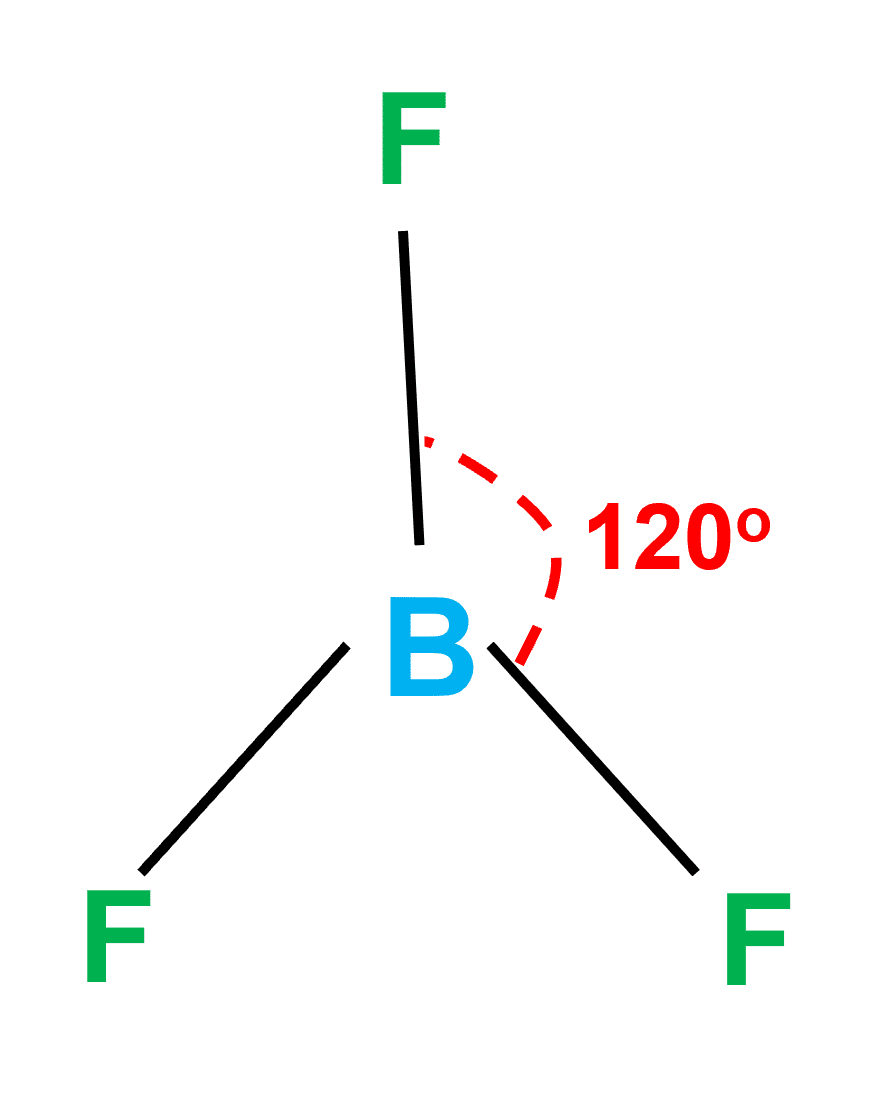
For SiF4, draw the Lewis structure, predict the shape, and determine if the molecule is polar or nonpolar. | Homework.Study.com