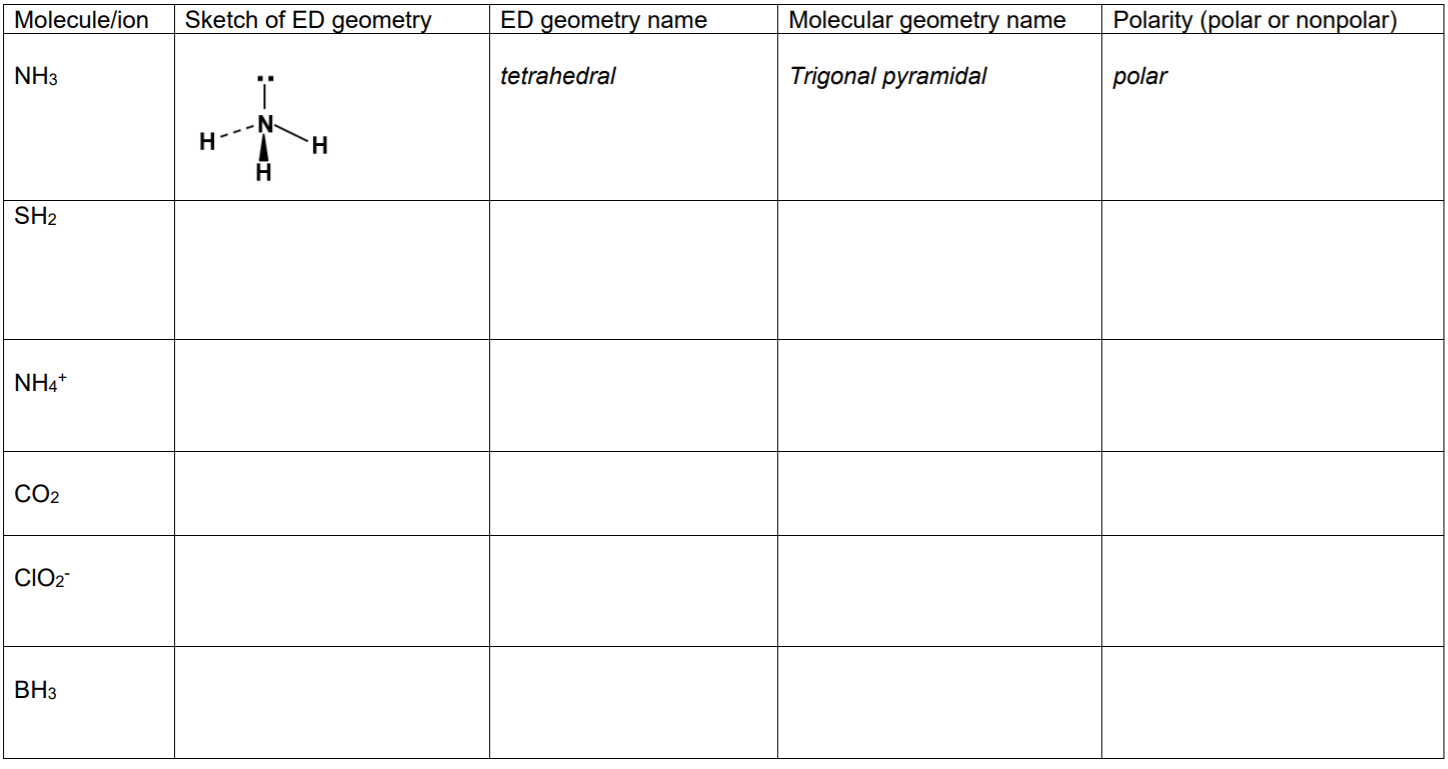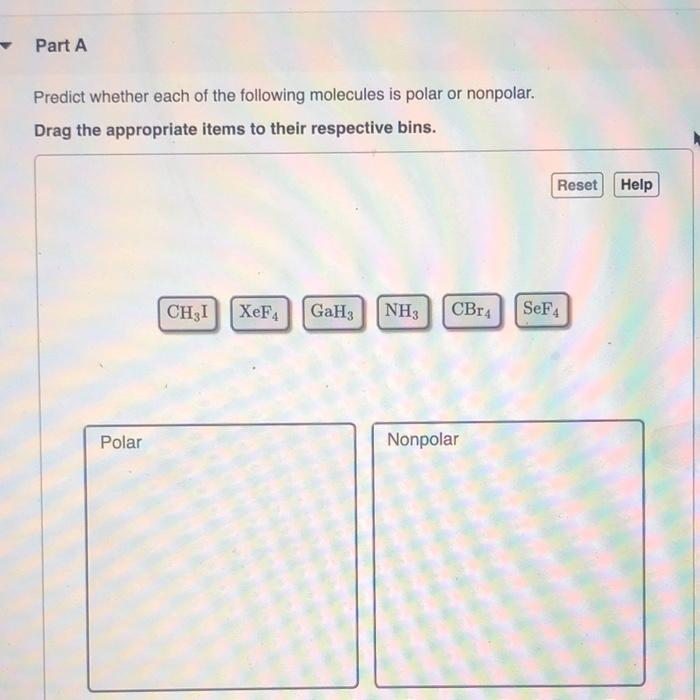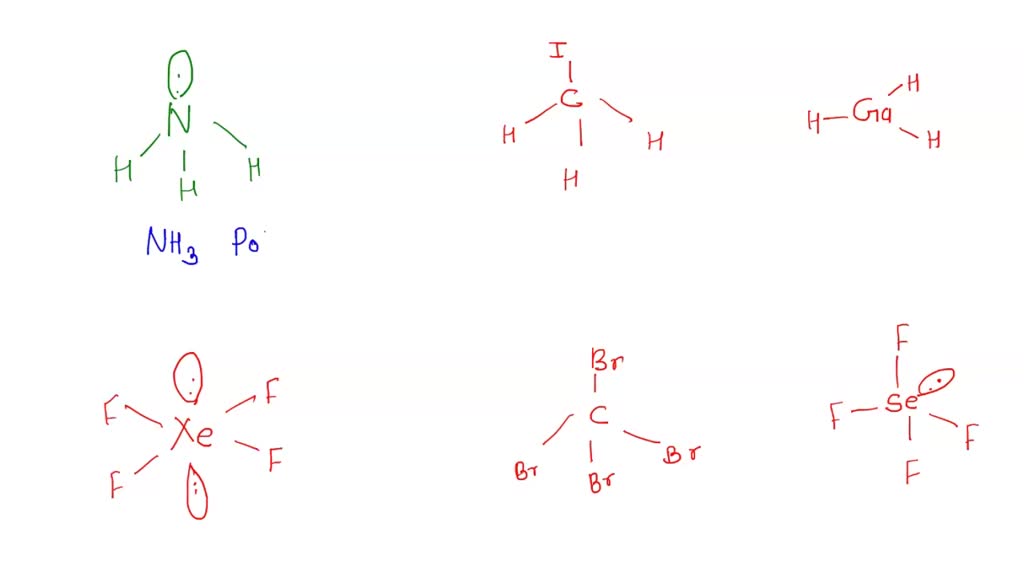
SOLVED: Part A Predict whether each of the following molecules is polar or nonpolar Drag the appropriate items to their respective bins Reset Help CH;l XeF4 GaH: NH; CBr4 SeF4 Polar Nonpolar

SOLVED: Choose the compound below that contains at least one polar covalent bond, but is nonpolar HCN IF3 SeF4 CBr4 Both B and C are nonpolar and contain a polar covalent bond.

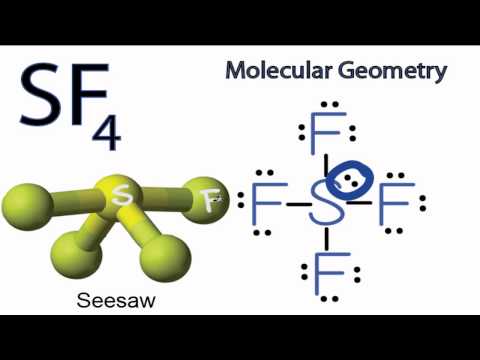
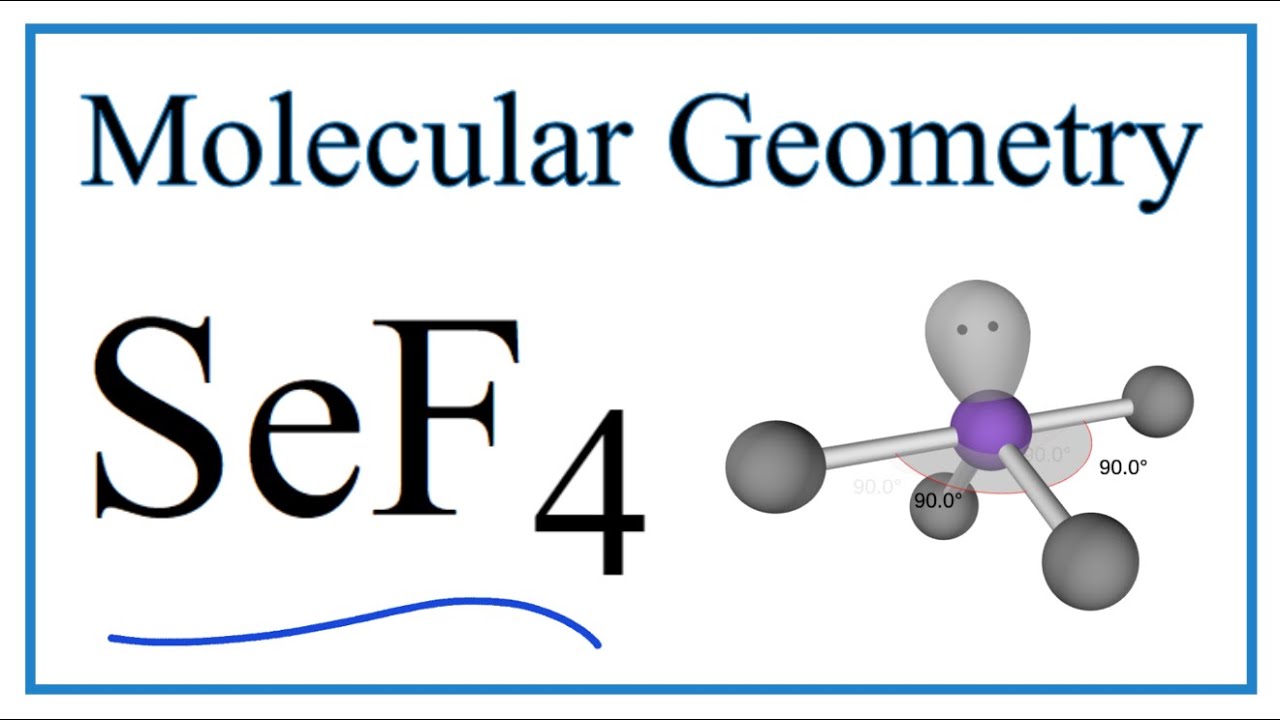
Consider the molecule SeF4. a. Draw the Lewis structure. b. What is the hybridization of Se? c. What is the electron geometry? d. What is the molecular geometry? e. What degree angles

Part A Predict whether each of the following molecules is polar or nonpolar Drag the appropriate - Brainly.com

SOLVED: Molecular Structure and Physical Properties Consider the following statements and determine which are true and which are false Bond angles for IF6 are 60 SeF4 has see-saw structure NO3 has only